+88 01712444336
Free Shipping when you spend 10,000 BDT
Go shop
Oxygen Cylinder Rent Service AVAILAVLE
RENT
Oxygen Concentrator Available
Concentrator
Login
Login
Register
+88 01712444336
Free Shipping when you spend 10,000 BDT
Go shop
Oxygen Cylinder Rent Service AVAILAVLE
RENT
Oxygen Concentrator Available
Concentrator
Menu
Categories
- Oxygen Concentrator Dhaka BD
- Dining & Bar
- Medical Oxygen
- Accessories & Others
- COVID-19 Products
- UV Sterilizer
- Lighting & Lamps
- Laboratory
- Roller Mixer
- Hematology Analyzer
- Rotator
- Hormone Analyzer
- Shaker
- Hot Air Oven
- Urine Analyzer
- Biochemistry Analyzer
- Lab Incubator
- Vortex mixer
- Blood Counter
- Laboratory Analyzer
- Water Bath
- Clinical Chemistry Analyzer
- Laboratory Centrifuge
- Coagulation Analyzer
- Laboratory Others
- Colorimeter
- Micro Plate Reader
- Electrolyte Analyzer
- Microscope
- Elisa & Microbiology Reader
- Platelet Rotator
- ESR Analyzer
- Food
- Pulse Oximeter
- Lighting & Lamps
- Orthopedic
- Appliances
- Traction Kits
- ELBOW HAND SUPPORT SPLINT
- Shoulder Supports
- Finger Splints
- Arm Clavicle Support
- Neoprene Support Braces
- Cast Care
- Knee Brace Support
- Knee Cap
- Ankle Support Braces
- Cervical Support Braces
- Foot Splint Walker
- Abdominal Support Braces
- Thigh and Calf Support
- Corset (Compression Garments)
- Varicose Vein Stockings
- Chest Support Braces
- Wrist & Forearm Splints
- Back Spinal Support Braces
- Fracture Aids
- Appliances
- Mattress & Accent
- Home Care Equipment
- ECG Accessories
- Hospital Bed – Furniture
- Urine Collector
- Dental Equipment
- Disposables Categories
- Medical Device
- Patient Bed Price in Bangladesh
- Physiotherapy Item
- Artificial Plants
- Medical Equipment
- Diabetes Machine BD
- Cabinetry
- Breathing & Respiratory Care
- Weighting Scale
- ECG – EEG – EMG
- CPAP and BiPAP Machine
- Eye Care
- Blood Pressure Machine
- Cabinetry
- Nebulizer Machine
- Dining & Bar
Wishlist
0
12 Channel ECG Machine Cell-G
Cell G ECG Machine for Clinical, Home, Hospital
| Technical Specifications | |
| Display | 4” touch screen |
| Language | English |
| Printout | On A4 Paper with Wireless printer |
| Storage | Storage of 10000 ECG, freedom of not taking a printout if not required |
| Printer | HP Wi-Fi enabled printer (Mopria supported) |
| Battery | Duracell 1.5V AA-type Nonrechargeable Requires 2 Duracell batteries |
| Input | 100-240V~50/60Hz 0.13 A |
| Output | 5.0V 1.0A |
| Dimension (in mm) | 180mmX118mmX52mm |
| Weight (in Kg) | Approx. 0.680kg |
| Operating Temp | Approx. 0.680kg |
| Operating & Storage | up to 95 % non-condensing |
12 Channel PC ECG Machine | Biocare ECG-2000
৳ 67,000.00PC ECG Biocare ECG-2000
The PC ECG Biocare ECG-2000 is delivered with the appropriate software, enabling a paperless workflow as ECGs can be measured and edited directly on the screen. The ECG is delivered as a complete operational set including cables and electrodes. You can also opt for a Biocare ECG-2000 set complete with a laptop.Product Details for the Biocare PC ECG
- PC ECG Biocare ECG-2000
- The pc-based, 12-lead resting ECG machine
- Real-time graphic representation
- ECGs directly measured and edited on the screen
- Easy reporting in all common data formats (BMP, JPG, Word, XML, Dicom)
- Comprehensive storage and printing options
- Data transfer and power supply via USB
Technical Details for Biocare ECG-2000
- High-performance chip with anti-drift technology
- High-performance data management software
- Pathological results are shown in color
- Sampling rate: 1,000 Hz
- A-D converter 24-bit
- The frequency response of 0.05 – 250 Hz, purpose-built for pediatric diagnostics
- Sensitivity: 5 mm/mV, 10 mm/mV, 20 mm/mV
- Analysis functions: VCG, VLP, HRV, HFECG, FCG, HRT and QT
System Requirements and Configuration
- Operating system: Windows 7 or later, Direct X9.0c, Drivers OFFICE 2000 Word or later
- Computer (recommended configuration): P4 2.0 GHz or higher; HDD: 80 GB or more
- The computer must meet the IEC 60950-1 guidelines
- Storage: 512 MB or more
- High-resolution color display: 1024 x 768 or better
- Printer: print resolution ≥ 600 dpi, laser printer or high-quality inkjet printers are recommended (do not use dot-matrix printers)
3 Channel ECG Machine- 303B
৳ 27,500.003 Channel ECG Machine- 303B
3 Channel ECG Machine- 303B is a very high-quality machine from ECARE, China.Specification:
| Brand | ECARE |
| Model | ECG -303B |
| Channel | 3 Channel |
| Country of Origin | China |
| Power supply | Adapt 110-240V, 50/60Hz AC |
| Multiple operation modes | Manual/Auto/Arrhythmia Analysis Modes |
6 Channel E600G electrocardiograph New!
৳ 0.006 Channel E600G electrocardiograph New!
Product Description:
- Dimensions of the camera (L x W x H): 295 x 225 x65 [ mm ]
- Simple, intuitive input and editing of patient, physician and hospital data
- 1600 Hz sampling • Direct operation of external A4 printers
- Constant measurement and display of HR
- Standard leads / Cabrera
- Semi-automatic analysis and interpretation
- Automatic analysis and interpretation
- Modes of activity: manual, automatic, rhythm-like.
- Print frequency adjustment-4 levels: low, moderate, ordinary and peak
- Dual power supply system: power supply line and battery: built-in impedance power supply 90 -240V
- VGA 800 x 480 (7 inches) color touch screen monitor for simple ECG graphs from selected leads
- Visual and audio signaling of unconnected electrodes
- Printing in 1, 3, 6 and 12 ECG waveforms
- Filters: 50Hz/60Hz network; Adaptive filter
- Print speed: 5; 10; 12.5; 25; 50 mm / s
- Sensitivity: 2.5; 5; 10; 20 mm / mV and AUTO
- Camera archive capacity: 1000 tests
- Camera weight:1.5-1.8 kg (depending on the camera version)
Available Equipment:
- E61 trolley
- ECG cable extension arm Options:
- Additional 2 USB ports, Pendrive support (SCP, HL7, PDF or PNG graphic format)
- Wireless (Wi-Fi) LAN or Internet communication
- Wired LAN or Internet communication
- Possibility to transfer ECG directly from the machine to your email inbox
Standard Equipment:
- ECG cable extension arm Options:
- Additional 2 USB ports, Pendrive support (SCP, HL7, PDF or PNG graphic format)
- Wireless (Wi-Fi) LAN or Internet communication
- Wired LAN or Internet communication
- Possibility to deliver ECG directly to your email inbox.
6 Channel ECG Machine- ECG-606A
৳ 45,000.006 Channel ECG Machine- ECG-606A
6 Channel ECG Machine- ECG-606A is a very high-quality machine from ECARE, China.| Product Name | ECG Machine |
| Brand | ECARE |
| Model | ECG-606A |
| Country of Origin | China |
| Power supply | Adapt 110-240V, 50/60Hz AC |
| Multiple operation modes | Manual/Auto/Arrhythmia Analysis Modes |
Acumen7-Polysomnograph with ECG
৳ 12,000.00Acumen7-Polysomnograph with ECG
Brand: Curative Model: acumen 7Specification:
- Acumen 7 is an asleep diagnostic device specifically designed for use in the home away from clinical environments.
- Up to 100 hours of appropriate physiological information can be recorded over up to 8 channels by the Acumen 7.
- Linked to its dedicated software, it offers instruments for displaying and analyzing recorded information and reporting for diagnosing respiratory sleep illnesses.
- The design of Acumen 7 ensures that the device is compact, light and comfortable to use.
- Technical specifications enable the medical team to diagnose the primary respiratory sleep disorders of the patient efficiently.
- Here is a tool particularly intended for it, which is Acumen 7, if you want a clinical atmosphere away from home.
Biocare 12 Channel ECG Machine
৳ 79,000.00
Biocare,12 Channel ECG Machine
Brand: Biocare
Model : BC-ECG-1210
Feature
- Biocare 12Ch Digital Resting ECG w/5.7 “LCD Foldable Screen, ECG-121
- Foldable monitor (0~80 degree) with 5.7 inches LCD, contrast is adjustabl
- R-R interval analysis, detailed and brief analysis reports meet different requirements
- Multi-language options
- Real-time waveform freezing
- Economy mode available help to reduce printing costs
- Long-lasting Li-ion battery works for up to 2 hours
- LAN port for real-time data transmission
- Easy upgrade keeps you updated
- Delightful operating experience with long-lasting silicone button, unique shuttle design
- FDA approved. Optional SD card, unlimited data storage
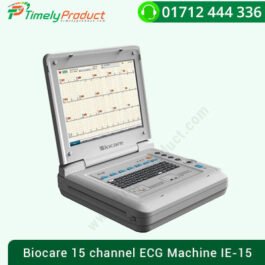
Biocare 15 channel ECG Machine IE-15
৳ 0.0015-CHANNEL ELECTROCARDIOGRAPH, IE-15, Biocare
| Characteristics | |
| Number of channels: | 15-channel |
| Technology: | computer-based |
| Options: | with display |
- 1-inch high brightness color touch-screen, handwriting support
- Alphanumeric keyboard with individual capital and numeric keys
- 1:1 ratio waveforms with grid line background can meet doctors ‘ diagnostic habits
- 15 leads simultaneously acquisition & 12 leads compatible
- Application of Minnesota Code, CSE & AHA database
- Cabrera display and report format available
- • Last 300 sec.
- R interval analysis, detection of arrhythmia, brief and detailed analysis reports
- VCG has more advantages in the diagnosis of the correct atrium and ventricular hypertrophy, conduction block, myocardial infarction, VBI coronary artery, and a pre-excitation syndrome
- Automatic printing after detection of arrhythmia
- ECG data can be saved as JPG, DICOM or XML format. Multiple ports for data transmission ( LAN, USB, SD card, VGA)
- Connection support of the ECG management system via WIFI (optional)
Biocare 3 Channel ECG Machine iE 300
৳ 45,000.00Biocare iE 300 ECG Machine
| Brand | Biocare |
| Model NO. | iE300 |
| Peripheral Ports | USB, LAN Port |
| Data Storage | Massive Local Storage Capacity up to 1500 Files |
| Recording Paper | 80mm Width |
| Origin | Jiangsu China |
Biocare IE-12A 12 Channel ECG Machine
৳ 0.00Biocare IE-12A 12 Channel ECG Machine
| Model NO | IE-12A |
| Trademark | BIOCARE |
| Transport Package | Carton |
| Classification | Physiological Functions of Diagnosis and Monitoring Equipment |
| Origin | Jiangsu China |
| Type | Digital EEG/ECG/EKG/EMG/VCG |
| Certification | CE, FDA |
| Group | Adult |
| Medical Device Regulatory Type | Type 1 |
| Power Supply | AC110-230V 50/60Hz |
Biocare IE-12A 12 Channel ECG Machine
৳ 95,000.00Biocare IE-12A 12 Channel ECG Machine
| Model NO | IE-12A |
| Trademark | BIOCARE |
| Transport Package | Carton |
| Classification | Physiological Functions of Diagnosis and Monitoring Equipment |
| Origin | Jiangsu China |
| Type | Digital EEG/ECG/EKG/EMG/VCG |
| Certification | CE, FDA |
| Group | Adult |
| Medical Device Regulatory Type | Type 1 |
| Power Supply | AC110-230V 50/60Hz |
Biocare Ie-6, 6-Lead ECG Machine
৳ 98,500.00Biocare Ie-6, 6-Lead ECG Machine
| Model NO | IE-6 |
| Trademark | Biocare |
| Origin | Shenzhen China |
| Classification | Physiological Functions of Diagnosis and Monitoring Equipment |
| Type | Digital EEG/ECG/EKG/EMG/ VCG |
| Certification | CE |
| Group | Middle-aged and Old |
| Medical Device Regulatory Type | Type 1 |
| Transport Package | Carton |
Biolite E30 3 Channel ECG with interpretation 80mm printer
Biolite E30 3 Channel ECG with interpretation 80mm printer
Functional requirements: an electric bedside device (AC-powered) designed to continuously detect, measure and display the electrocardiogram (ECG) of a patient by means of leads and sensors attached to the patient, and typically display heart rate. Usually, the system is fitted with audible and/or visual warnings activated when the parameters of the patient fall below or exceed pre-set limits. Continuous display of patient ECG and heart rate on screen, Allows display of single, 5 lead ECG or simultaneous display of at least 5 waves selected from up to 12 points. The operator can set audiovisual alarm levels for low or high heart rate. Operates from mains voltage or from the internal rechargeable battery.BioLite E70 Digital 12-Channel ECG Machine
BioLite E70 Digital 12-Channel ECG Machine
Features:
The electrocardiograph can store 100 groups of ECG data in XML format with an optional SD card or U disk memory space in automatic background processing mode. Data reports as a thermal dot array with a horizontal and vertical resolution of 40 dots / mm, 8 dots / mm, 5 mm / s, 6.25 mm / s, 10 mm / s, 12.5 mm / s, 25 mm / s, 50 mm / s with a deviation of ±3 percent and a linearity of 0.5 mm/30M. The reel Thermo and sensitive thermo-sensitive printing paper enables 295mm/280 mm long and 210mm/216 mm wide paper to be printed in data. The computer measures 400x330x146 mm weighs approximately 6Kgs and displays 8.0 “color TFT LCD with 800* 600 pixels.BPL Cardioline ECG100+
৳ 0.00BPL Cardioline ECG100+ 6 Channel ECG Machine
Cardioline 100+ is a sleek and stylish six-channel ECG recorder designed with contemporary needs in mind. In combination with robustness and reliability, 100 + offers traces that you can trust, Equipped with an alphanumeric keypad and a color display. Equipped with Glasgow algorithm, it accurately interprets ECGs and comes with various communication protocols and various information transfer formats. Technical Specifications| ECG Leads | 12-leads (I, II, III, aVR-L-F, V1-6) |
| CMRR | 115 dB |
| Sampling Frequency | 500 or 1000 Hz/channel/storage |
| A/D Conversion | 24 bit |
| Resolution | < 1uV/LSB |
| Bandwidth | 0.05 – 150 or 300 Hz depending on the sampling rate |
| Pacemaker Detection | Hardware and software correlated detection with digital filter |
| Filters | Fully digital high-pass diagnostics filter; digital AC interference adaptive filter (50/60 Hz); digital low-pass filter (applied during display and printing stage) |
| Defibrillation Protection | AAMI/IEC standards |
| Front-End Performance | ANSI/AAMI IEC 60601-2-25:2011 |
| Safety | ANSI/AAMI ES1, Class I CF, CE0476 |
| ECG Storage | Internal memory 100 ECGs, an expansion for 1000 additional ECGs (optional) |
| Display | 4.3″ backlit LCD color display, displays the ECG waveform in real time |
| Thermal Printer | 8 dot/mm; 108mm; Z-fold 150mm paper |
| Manual Printing | 3 or 6 channels -5/10/25/50 mm/s |
| Automatic Printing | Standard or Cabrera; 3, 3+1, 6 channels |
| Patient Demographic, Global Measurements, Optional Interpretation (Glasgow University – Prof. MacFarlane) | |
| Keypad | Mechanical keypad with alphanumeric keys and function keys |
| Connectivity | USB device, LAN, 802.11g/n Wi-Fi (optional) |
| Patient Cable | Standard 15D, 10 electrodes |
| Data Export | PDF, XML (optional), GDT (optional), DICOM (optional), HL7 (optional) |
| Power Supply | Medical AC power-supply unit (100-240 VAC 50/60 Hz); internal rechargeable battery |
| Dimensions | 285 x 204 x 65 mm |
| Weight | 1.8kg with battery |
Cardioline 200+ 12-Channel ECG
৳ 0.00Cardioline 200+ 12-Channel ECG
The Cardioline ECG200 + is the recent developed 12-lead ECG channel for displaying, acquiring, printing (on A4 z-fold paper) and storing ECG examinations. Technical Specifications- ECG leads — 12-leads (I, II, III, aVR-L-F, V1-6)
- CMRR — > 100 dB
- DC input impedance — No lead-off 100 M
- A / D converter — 24 bit, 32 kHz
- Sampling frequency — 500 Hz
- A / D conversion — 20 bit
- Resolution — < 1uV / LSB
- Bandwidth – 0.05 – 150 Hz
- Pacemaker detection – hardware detection combined with digital filtering convolution
- Filters – Fully digital high-pass filter diagnostics ; adaptive digital AC interference filter (50/60 Hz) ; digital low-pass muscle filter 25 and 40 Hz (only for screen and printing)
- Protected defibrillator – AAMI / IEC standards
- Front-end performance – ANSI / AAMI IEC 60601-2
- ECG storage — internal memory 100 ECGs, expansion for 1000 additional ECGs (optional)
- Display — 7 “backlit LCD color display, display of the ECG wave in real time
- Thermal printer — 8 dot / mm ; 216 mm ; A4 Z-fold
- Manual printing — 3, 6 or 12 channels — 5/10/25/50 mm / s
- Automatic printing — Standard or Cabrera ; 3, 3 + 1, 3 + 3, 6 or 12 channels, Patient Demographic, Global measurements, Optional Int.
- Weight 2.6 kg with battery
- Domination 396x290x80
COMPUTERISED ECG, Complete on Trolley
৳ 0.00COMPUTERISED ECG, Complete on Trolley
| DEVICE NAME: | COMPUTERISED ECG, Complete on Trolley |
| Model Number: | ClECG 01 |
| Brand Name: | BIBEAT LTD |
| Origin: | Bangladeshi |
| Weight: | 25 Kg |
| Warranty: | 24 Months |
- Standard 12 Diagnostic ECG with 10 lead patient cable
- PC-based data storage and printing facility Friendly graphical user interface [ GUI ]
- Single channel data acquisition
- Single channel data acquisition
- Individual leads selected with simple mouse clicks on GUI
- Stand-alone and telemedicine
- Combined 12 lead ECG graph in pdf format
- Runs on Windows XP and later version.
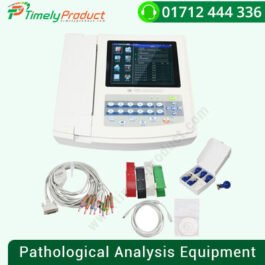
CONTEC 8000G 12 Lead ECG workstation mini size EKG machine connected with computer
৳ 35,000.00CONTEC 8000G 12 Lead ECG workstation mini size EKG machine connected with computer
| Type | Pathological Analysis Equipment |
| Brand Name | Contec |
| Place of Origin | Hebei, China |
| Model Number | 8000G |
| Instrument classification | Class II |
| Product name | Stress test ecg machine |
| Lead | Standard 12 leads |
| Noise level | ≤15μVp-p |
| Safety type | Class II Type CF with defibrillation protection |
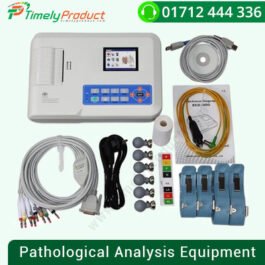
CONTEC ECG 1200G Touch Screen 12-Channel ECG Machine
৳ 74,000.00CONTEC ECG 1200G Touch Screen 12-Channel, 12 Lead Electrocardiograph Machine
| Type | Pathological Analysis Equipment |
| Brand Name | CONTEC |
| Place of Origin | Hebei, China |
| Model Number | ECG1200G |
| Instrument classification | Class II |
| Name | CONTEC ECG |
| Trade | Trade assurance service provided |
| Weigh | 3.2Kg |
| Dimension | 340mm(L) * 320mm(W) * 85mm(H) |
| Power supply | AC/DC |
| AC | 100V~240V(50/60Hz) |
| DC | 14.8V/3500mAh rechargeable lithium battery |
| Paper size | 210mm(W)*20m(L) |
| Certificate | CE ISO FDA |
CONTEC ECG 1200G Touch Screen 12-Channel Elecreocardiograph
৳ 74,500.00CONTEC ECG 1200G Touch Screen 12-Channel Elecreocardiograph
| Type | Pathological Analysis Equipment |
| Brand Name | CONTEC |
| Place of Origin | Hebei, China |
| Model Number | ECG1200G |
| Instrument classification | Class II |
| Name | CONTEC ECG |
| Trade | Trade assurance service provided |
| Weigh | 3.2Kg |
| Dimension | 340mm(L) * 320mm(W) * 85mm(H) |
| Power supply | AC/DC |
| AC | 100V~240V(50/60Hz) |
| DC | 14.8V/3500mAh rechargeable lithium battery |
| Paper size | 210mm(W)*20m(L) |
| Certificate | CE ISO FDA |
CONTEC ECG 300G Portable 3 Channel 12 Lead EKG Portable ECG Machine
৳ 29,500.00CONTEC ECG 300G Portable 12 Lead EKG Portable ECG Machine
| Brand Name | CONTEC |
| Model Number | ECG300G |
| Place of Origin | Hebei, China |
| Instrument classification | Class II |
| Type | Examination Therapy Equipment |
| Certificate | CE ISO FDA SFDA ROHS |
| Product name | 12 Lead ECG 3 channel |
| Weigh | 1.6Kg |
| Dimension | 315mm(L) *215mm(W) * 77mm(H) |
| Display | 3.5″ TFT Color LCD display |
| Optional | The software which can transfer data to PC |
| Multi-language | interface and report |
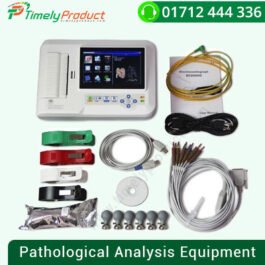
CONTEC Touch 6-Channel Elecreocardiograph 12-Lead ECG/EKG Machine + PC Software, ECG 600G
৳ 44,500.00CONTEC Touch 6-Channel Elecreocardiograph 12-Lead ECG/EKG Machine + PC Software, ECG 600G
| Type | Visual Acuity Examination Apparatus |
| Brand Name | CONTEC |
| Place of Origin | Hebei, China |
| Model Number | ECG600G |
| Instrument classification | Class II |
| OEM/ODM | Yes |
| Certificate | CE |
| Power supply | AC/DC |
| Product Name | 6 channel electrocardiograph ECG machine |
| Lead | 3/6/12-lead display,12-lead Sync collection |
| Multi-language | interface and report |
| Display | 7″TFT-LCD(touch screen) |
Digital 12 Channel ECG Machine Medigate Me CA 812i
Digital 12 Channel ECG Machine Medigate Me CA 812i
Model: Me CA 812i Brand: Medigate Origin: Korea Medigate 12 Ch Eletro cardiograph, 12 leads Resting Template MeCA812i ECG Analysis Device. With default Medigate Corp. Korea accessories. Features:- Built-in high-resolution 8-inch thermal printer
- Rechargeable battery
- Software management device [ PC Interface: RS232, LAN(reserve) ]
- Complete alphanumeric keyboard capability [ Option: PS2 keyboard ]
- ECG space [ Typical: 50ECGs, Option(1 GB SD card): 5000 ECGs ]
- Firmware upgrade via USB memory stick
- 320 * 204(dots) Graphical LCD screen.
- High Quality, Easy-to-use, and Convenience
- Purchase, review, processing, and printing one-button process.
- The ECG signal’s LCD preview shows a misleading lead and signals distortion.
- Complete alphanumeric keyboard for convenient operation.
- Continuous recording of 3,6,12 channels in real-time.
- Me CA 812i will store up to 5000 ECGs with the SD-Card.
- Printing, printing, and distribution of ECGs .
- Using the RS232, LAN or SD card, ECG can be transmitted from the ECG cardiograph to the PC (Mecalyzer).
- The capability of server/client network service
- Image (Jph or BMP) capability to capture printing.
ECG Machine 12 Channel iMAC-120 (Zoncare)
৳ 0.00Zoncare 12 Channel ECG Machine
Model: iMAC-120, Zoncare| Type | Pathological Analysis Equipment |
| Brand Name | Zoncare |
| Place of Origin | Guangdong, China (Mainland) |
| Model Number | iMAC120 |
| Instrument classification | Class II |
| Product name | iMAC120 portable price of ECG machine 12 channel |
| Display | 10 inch HD LED Touch display |
| Channel | 12-ch resting ECG |
| Printing mode | Manual, Auto 3*4, 3*4+R, 3*4+3R, 6*2,6*2+1R,12*1 |
| Measurement | Auto measurement and analysis, ECG reply |
| Printing | Print A4 report by USB connecting with a Laser printer |
| Storage | 1000 data built-in storage, support SD card or USB extended |
| Other function | Qwerty keyboard and short cut function key input |
| Size | 395mmx240mmx420mm |
| Warranty |
12 months |
Edan SE-1200 Express Basic 12-Channel ECG Machine
৳ 0.00Edan SE-1200 Express Basic 12-Channel ECG Machine
| Product Name | ECG Machine |
| Model | SE-1200 Express |
| Brand | Edan |
| Number Of Channels | 12 |
| Dimension | 420x330x120 mm |
| Power Supply | 100-240 V,50/60 Hz |
| Battery Type | 14.8 V Rechargeable Built-In Li-ion Battery |
| Paper Width | 216,210 mm |
| Record Paper | Roll and Z-Fold |
| Display | 10.1 inch LCD Screen |
| Type | Digital |
| Lead Selection | Automatic |
Electroencephalograph (EEG)_VIRGO – 24
৳ 0.00Electroencephalograph (EEG)
Brand: ALLENGERS Models: VIRGO – 24 Country origin: India Allengers Virgo series of EEG test devices are intended using state-of-the-art technology and brain electrical activity recording and tracking and seizure origin investigation and location. Allengers EEG highlights advanced digital signal processing and brain mapping methods. Special features:- USB plug & play with Portable EEG (external supply is not required).
- Lightweight & compact electrode amplifier of high performance.
- Integrated Electrode Connection Impedance Check.
- Features such as AEEG: Amplitude Integrated EEG, CSA / DSA frequency distribution, • Brain Mapping etc.
- Optional–MPEG-4 high-resolution synchronized video with EEG recording.
- Editable User Unlimited number of installations.
- Facility to send EEG information in MATALAB / Lab view / Media Player Format via e-mail and export.
- Free Lifetime Online Support & S / W upgrade
- Converter integrated with EDF (European Data Format).
- Completely portable and simple to use on the ICU / Bedside.
- USB / ethernet interface allows procurement stations based on desktop or laptop.
- Flexible LED-based photonic stimulator.
- User-friendly and easy to use with many other characteristics.
- Optional SPO2,HR & Pleth Recording Facility
- Battery, High-End Camera (Pan, Tilt & Zoom) optional.
Warranty: 1 Year
ETT Machine RMS Vega 201 Treadmill Test (TMT)
৳ 0.00RMS Vega 201 Treadmill Test (TMT)
| Product Name | RMS Vega 201 Treadmill Test (TMT) |
| Model | VEGA 201 |
| Display Type | Digital |
| Grade | Semi-Automatic |
| Brand | RMS |
| Supply | AC |
| Minimum Order Quantity | 1 Unit |
Warranty: 1 Year.
Fukuda Cardimax FX-8222 ECG Machine
৳ 0.00Fukuda Cardimax FX-8222 ECG Machine
Features:
- Makes a wide range of applications
- Wide 6.4 inch Color TFT monitor
- Touch screen activity
- 3/6/12 Lead ECG display
- 3/6/12 channels recording on 145 mm paper
- Varieties of recording formats
- Internal memory for ECG storage
- Network connection to Ethernet LAN port
- Wireless LAN compatible
- Roll and Z-fold paper compatible
- Update to Interpretation System (Optional)
- Complete keyboard.
- Dimensions: 307 (width) x 220 (deep) x 65 (height) mm
- Weight: 3 kg (battery-free)
FUKUDA DENSHI Advanced Electrocardiograph FX-8200
৳ 0.00FUKUDA DENSHI Advanced Electrocardiograph FX-8200
Specifications
- 12 months manufacturer’s warranty
- Accessory pack
- Interpretation / Arrhythmia function
- 800 x 480 resolution colour LCD display
- 6 / 12 lead ECG display
- 6 / 12 channel on 110 mm paper
- Internal memory for ECG data storage
- LAN port for external connectivity
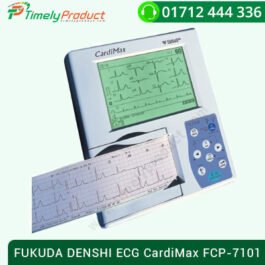
FUKUDA DENSHI ECG CardiMax FCP-7101
৳ 90,000.00FUKUDA DENSHI ECG CardiMax FCP-7101
This Fukuda Denshi ECG weighs only 1.2 kg and is smaller than an A4 sheet norm. Nevertheless, it offers a wide range of applications and high performance. ECG :- 12 Standard leads
- Frequency response: 0.05Hz to 150 Hz (within -3 dB)
- Filters: AC:50 or 60 Hz
- Muscle: 25 or 35 Hz
- Drift: 0.25 or 0.5 HZ 6y660z
- Interpretation: Brugada Criteria
- Definition of Arrhythmia,
- Explanation of Criteria for Evaluation
Categories
- Accessories & Others
- Artificial Plants
- Blood Pressure Machine
- Breathing & Respiratory Care
- Cabinetry
- Cabinetry
- COVID-19 Products
- CPAP and BiPAP Machine
- Dental Equipment
- Diabetes Machine BD
- Dining & Bar
- Dining & Bar
- Disposables Categories
- ECG – EEG – EMG
- ECG Accessories
- Eye Care
- Food
- Home Care Equipment
- Hospital Bed – Furniture
- Laboratory
- Biochemistry Analyzer
- Blood Counter
- Clinical Chemistry Analyzer
- Coagulation Analyzer
- Colorimeter
- Electrolyte Analyzer
- Elisa & Microbiology Reader
- ESR Analyzer
- Hematology Analyzer
- Hormone Analyzer
- Hot Air Oven
- Lab Incubator
- Laboratory Analyzer
- Laboratory Centrifuge
- Laboratory Others
- Micro Plate Reader
- Microscope
- Platelet Rotator
- Roller Mixer
- Rotator
- Shaker
- Urine Analyzer
- Vortex mixer
- Water Bath
- Lighting & Lamps
- Lighting & Lamps
- Mattress & Accent
- Medical Device
- Medical Equipment
- Medical Oxygen
- Nebulizer Machine
- Orthopedic
- Appliances
- Abdominal Support Braces
- Ankle Support Braces
- Arm Clavicle Support
- Back Spinal Support Braces
- Cast Care
- Cervical Support Braces
- Chest Support Braces
- Corset (Compression Garments)
- ELBOW HAND SUPPORT SPLINT
- Finger Splints
- Foot Splint Walker
- Knee Brace Support
- Knee Cap
- Neoprene Support Braces
- Shoulder Supports
- Thigh and Calf Support
- Traction Kits
- Varicose Vein Stockings
- Wrist & Forearm Splints
- Fracture Aids
- Appliances
- Oxygen Concentrator Dhaka BD
- Patient Bed Price in Bangladesh
- Physiotherapy Item
- Pulse Oximeter
- Uncategorized
- Urine Collector
- UV Sterilizer
- Weighting Scale
Filter by price
You may like it
-
 ৳ 1,658.00
৳ 1,658.00 -

Dental Curing Light Optic Fiber
৳ 999.00 -